AASLD Consensus Expert Panel Statement on the Clinical Best Practice in Managing Liver Patients in Times of COVID-19 Pandemic
COVID Patient
LIVERFASt™ can safely provide the complete liver assessment that is clinically needed, whether a COVID-19 patient has pre-existing conditions like hepatitis B or C, NASH or Fatty Liver, or even those with no diagnosed liver disease but with the presence of Metabolic Syndrome, Type 2 Diabetes or Obesity.
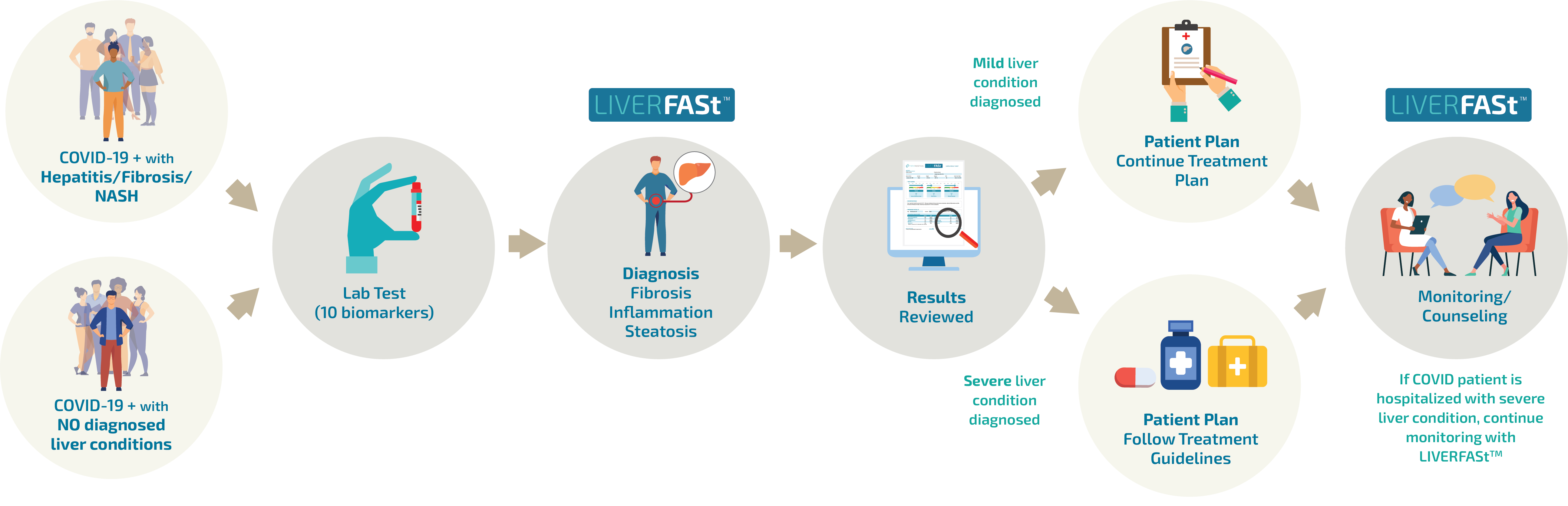
How LIVERFASt™ helps in COVID-19?
COVID-19 infection potentially targets the liver, with patients experiencing various degrees of liver function abnormalities. The liver is a concern for COVID-19 and clinicians need to determine whether liver injury is related to underlying liver diseases, drugs to treat direct effects of the virus, or a complicated disease course.
With chronic liver disease being associated with developing more severe outcomes for COVID-19, LIVERFAStTM can help COVID-19 patients to quickly identify liver conditions like fibrosis, inflammation and steatosis to prevent severe outcomes. Patients can simply go to the lab and have blood collected for diagnosis with LIVERFAStTM , avoiding any potential COVID-19 exposure in hospital settings.

Learn more from









